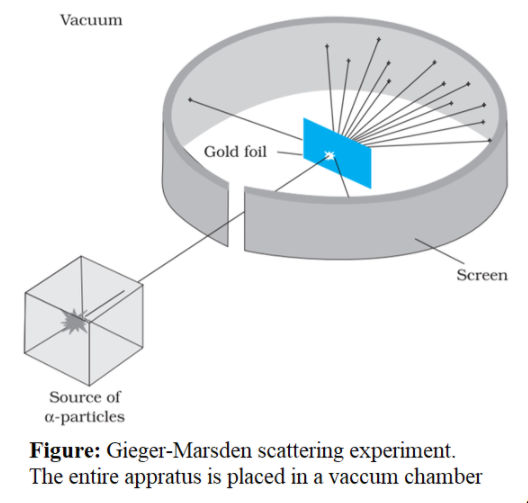
Gold Foil Experiment Images . Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom. Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil.
from www.animalia-life.club
Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom.
Rutherford Gold Foil Experiment
Gold Foil Experiment Images Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom.
From www.britannica.com
Ernest Rutherford Atomic Theory, & Facts Britannica Gold Foil Experiment Images Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom. Web learn how rutherford, geiger and marsden discovered. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom. Find out the definition, parts, and shortcomings. Gold Foil Experiment Images.
From www.animalia-life.club
Rutherford Gold Foil Experiment Gold Foil Experiment Images Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web learn how rutherford, geiger and marsden discovered the. Gold Foil Experiment Images.
From gcsephysicsninja.com
1. Rutherford's gold foil experiment Gold Foil Experiment Images Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford's gold foil experiment led. Gold Foil Experiment Images.
From www.youtube.com
Rutherford's Gold Foil Experiment Quick and Simple! YouTube Gold Foil Experiment Images Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment. Gold Foil Experiment Images.
From www.shutterstock.com
Rutherford Gold Foil Experiment Infographic Diagram Stock Vector Gold Foil Experiment Images Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment. Gold Foil Experiment Images.
From www.alamy.com
Rutherford goldfoil experiment Stock Photo Alamy Gold Foil Experiment Images Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Find out the definition, parts, and shortcomings of the. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Find out the definition, parts, and shortcomings of the model, and how it differs from the. Gold Foil Experiment Images.
From www.animalia-life.club
Rutherford Gold Foil Experiment Gold Foil Experiment Images Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal. Gold Foil Experiment Images.
From www.youtube.com
Rutherford's Gold Foil Experiment, Explained — Discovering the Nucleus Gold Foil Experiment Images Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how rutherford's gold foil experiment led to the nuclear model of the atom, which has a small, dense nucleus and a cloud of electrons. Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom. Web learn how rutherford's gold foil experiment led to the nuclear model of the. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Find out the definition, parts, and shortcomings of the model, and how it differs from the modern atomic theory. Web learn how rutherford, geiger and marsden discovered the atomic nucleus by scattering alpha particles on a gold foil. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the. Gold Foil Experiment Images.
From
Gold Foil Experiment Images Web a sketch of the layout of rutherford's experiment, in which he bombarded a slice of gold with alpha particles to reveal the composition of the atom. Web learn how ernest rutherford and his student ernest marsden conducted the gold foil experiment that led to the creation of the rutherford atomic model. Web learn how rutherford's gold foil experiment led. Gold Foil Experiment Images.